CIVO® Platform
How CIVO Works
In a few simple steps, this patented process can simultaneously evaluate multiple drugs or drug combinations using 100+ assays for tumor and microenvironment responses.



Civo evaluates clinical response
where it matters most—the patient's tumor
FDA, IRB, OR IACUC-Approved Research
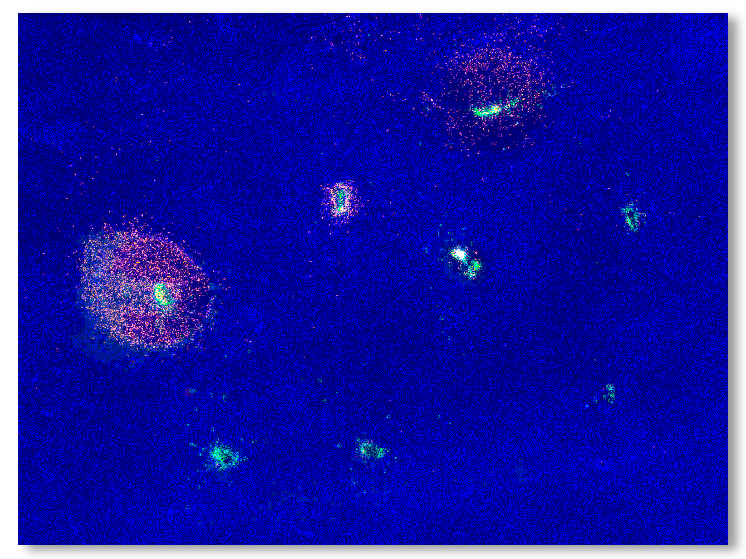
CIVO Microdose Injections induce anti-tumor immune response
Fundamentally changing cancer
drug development
In a few simple steps, this patented process can simultaneously evaluate multiple drugs or
drug combinations using 100+ assays for tumor and microenvironment responses.
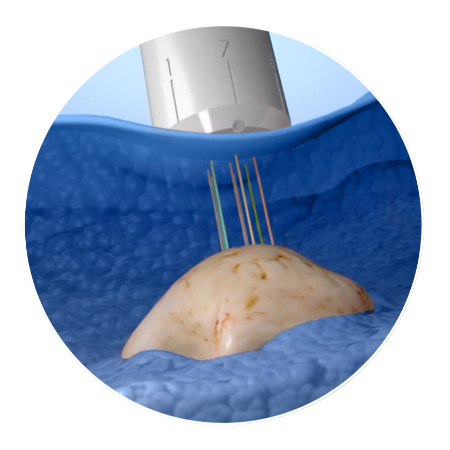
CIVO injects drug microdoses directly into a patient's tumor. The device delivers trackable drug "columns."
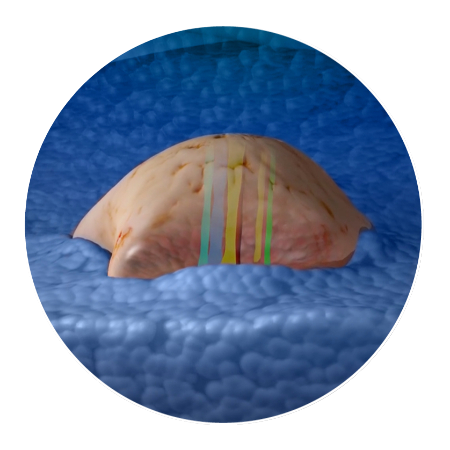
Drugs interact with the tumor while still within the patient. Then the tumor is surgically removed.
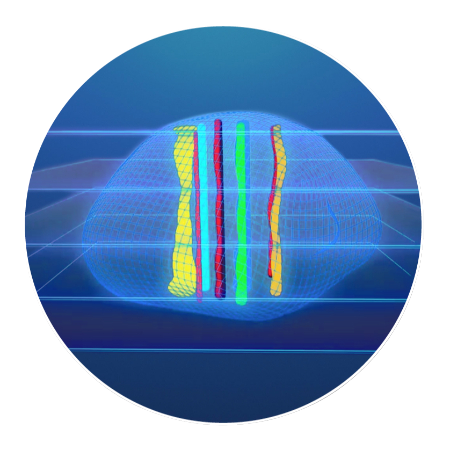
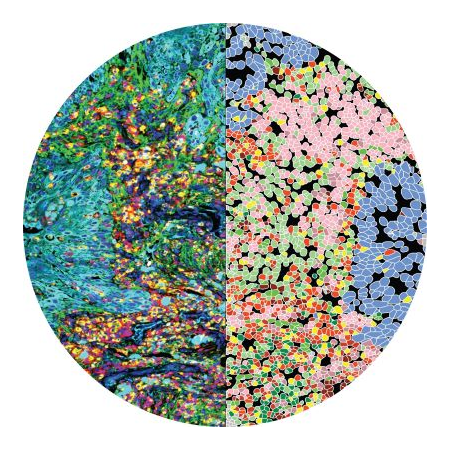
The tumor tissue is processed by sectioning crosswise to the drug columns. Four-micron sections are then cut and placed onto slides for staining.
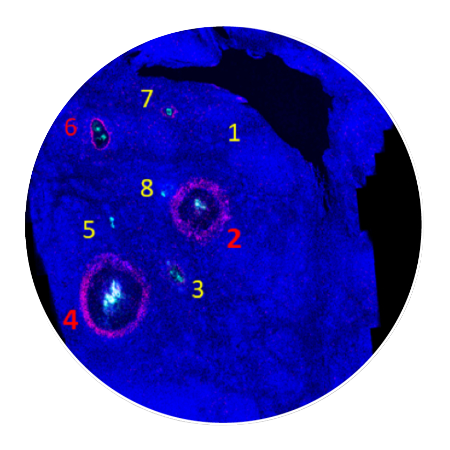
Tumor slices are assessed for response around each injection site using CIVOanalyzer
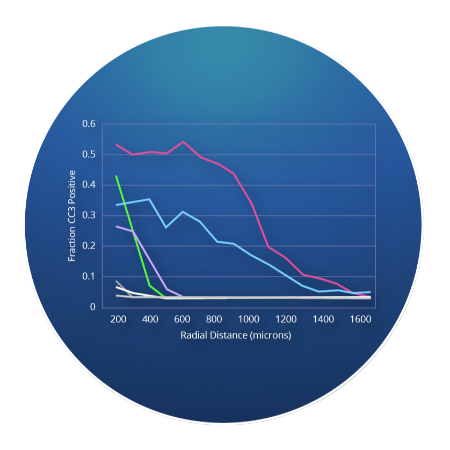
CIVOanalyzer ™ delivers automated response quantification via radial distance calculations, effectively comparing responses between drugs and drug combinations.
Fundamentally changing cancer drug development
In a few simple steps, this patented process can simultaneously evaluate multiple drugs or drug combinations using 100+ assays for tumor and microenvironment responses.
CIVO: designed to solve a long-standing challenge