Clinical Collaboration
Gain critical
Innovation in Drug Development: CIVO Phase 0 Clinical Trials
What is “Phase 0”?
According to the United States Food and Drug Administration, Phase 0 studies, also known as exploratory IND studies, are conducted in humans however unlike Phase I-IV studies, they typically involve very limited patient exposure to investigational agents and have no therapeutic or diagnostic intent.
Phase 0 studies can help identify, early in the process, promising candidates for continued development and eliminate those lacking promise. Presage is uniquely positioned to execute on exploratory IND studies because of our CIVO platform: Instead of systemic administration, microdoses of drugs are injected intratumorally and our CIVO GLO ensures we can track drug exposure. Directly interrogating the human tumor microenvironment provides the clearest picture of drug mechanism of action.

Gain critical
Insights earlier in the oncology drug development process
Traditional mouse models can show encouraging data, but these responses often fall short in later-stage trials. Presage’s CIVO® (Comparative In Vivo Oncology) microdosing technology delivers quantitative evidence of a drug’s effects on a patient’s tumor in the Phase 0 setting.
A bridge between cancer biology and clinical responses
Clinical development of novel oncology agents takes years, costs millions of dollars, and the likelihood of FDA approval is less than 10%.
Phase 1 trials provide preliminary safety and pharmacokinetic data, but how a patient’s cancer will respond and the effect of investigational agents on the tumor are still unclear. Often, the anti-tumor drug activity observed in laboratory and mouse models are not sustained when patients are treated with the same agents during clinical trials. Preclinical tests are unable to replicate the complexities and unique behaviors of the human immune system and tumor microenvironment.
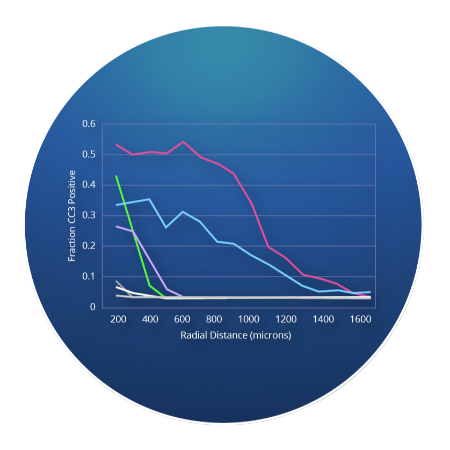
Presage is pioneering a new early oncology drug development process using patented CIVO technology. CIVO simultaneously evaluates microdoses of multiple drugs or drug combinations in a patient’s tumor to determine drug responses and their impact on the tumor microenvironment.
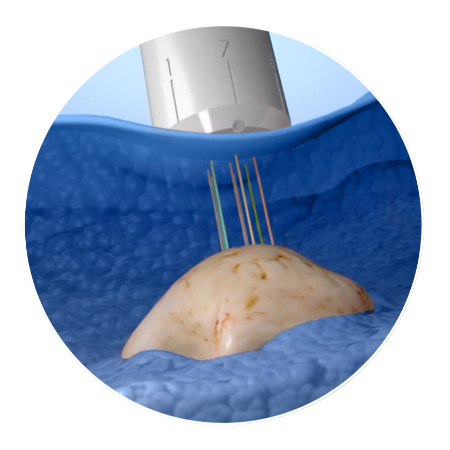
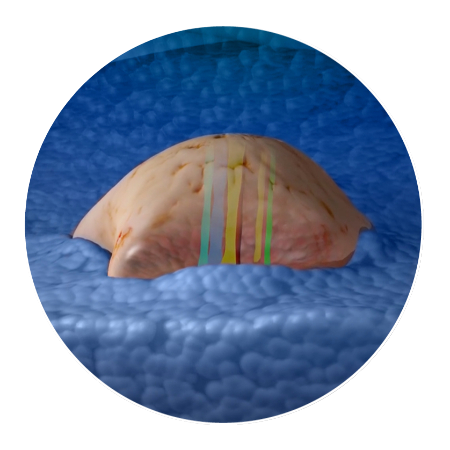
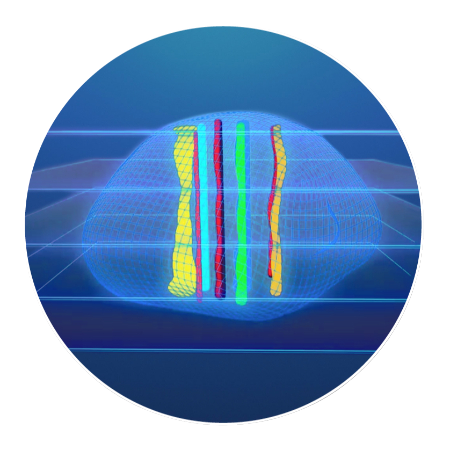
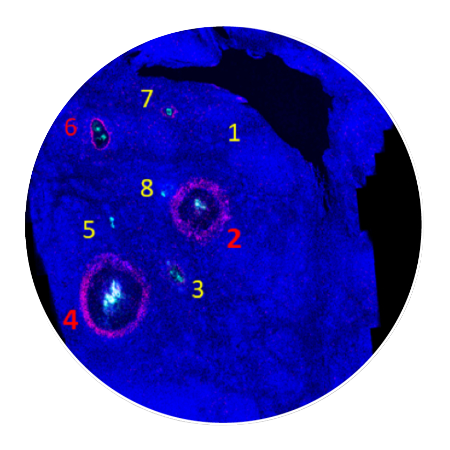
How CIVO Works
In a few simple steps, this patented process can simultaneously evaluate multiple drugs or
drug combinations using 100+ assays for tumor and microenvironment responses.
Clinical development of novel oncology agents takes years, costs millions of dollars, and the likelihood of FDA approval is less than 10%.Phase I trials provide preliminary safety and pharmacokinetic data, but the investigational effect on the tumor is still unclear.Often, the anti-tumor drug activity observed in laboratory and mouse models are not sustained when patients are treated with the same agents during clinical trials, because preclinical tests are unable to replicate the complexities and unique behaviors of the human immune system and tumor microenvironment.
Presage is pioneering a new early oncology drug development process using patented CIVO® technology. CIVO simultaneously evaluates microdoses of multiple drugs or drug combinations in a patient’s tumor to determine drug responses and their impact on the tumor microenvironment.
CIVO is intended to:
- Save development time with rapid analysis
- Evaluate multiple drug candidates and combinations in parallel, providing insights on mechanism of action, efficacy, and immune modulation of a drug based on real tumor microenvironment response
When evaluating multi-drug combinations
- The synergistic mechanisms can be elicited, rather than extrapolating from the proposed additive effects
- Resistance pathways can be evaluated to identify optimal drug combinations
- Reduce patient exposure to potentially toxic drugs
- Prioritize more promising drug candidates and combinations to accelerate development timelines and efficiently use valuable resources
Obtaining results with CIVO means more time focused on the investigational assets that hold the most promise for patients.

FOR ALL OF OUR PARTNERS
A Highly Collaborative Approach
Our team is experienced in all aspects of the clinical drug development process—from operations and scientific leadership to quality assurance and regulatory support. We integrate seamlessly into your drug development process so you can continue focusing on developing life-saving therapies.
CIVO Clinical Trials
MST01-TAK-02: TAK-676 Alone and in Combination(s) with Carboplatin, 5-Fluorouracil, and Paclitaxel in Patients with Head
and Neck Squamous Cell Carcinoma![]()

A defined regulatory pathway
In 2006, the FDA recognized the potential of Phase 0 trials and released guidance for industry on microdosing studies. Since then, the agency has simplified the process for using exploratory investigational new drugs which aligns perfectly with the CIVO platform’s capabilities.
The Center for Drug Evaluation and Research and Center for Devices and Radiological Health recognize CIVO as an accepted research tool. To allow for clinical trial efficiencies, the Master Access File for CIVO can be cross-referenced for any new trial that uses CIVO.
See how CIVO is helping to advance the science of oncology drug innovation.